Factors affecting entropy
1)change in tempearature
-entrpy increases as temperature increases
2)change in number of gaseous particles
-entropy increases as the number of gaseous particles increases.
3)change in phase
-entropy increases as solid changes to liquid and to gaseous state.
4)mixing of particles
-entropy increases when two pure gases are mixed and allowed to diffuse into each other.
-entropy increases when a solid dissolves in a liquid.
Tuesday, September 1, 2009
Sunday, August 23, 2009
chemistry
State of phosphorus: solid
state of sulfur:solid
TREND OF MELTING POINT FOR ELEMENTS IN PERIOD 3:
Melting points generally increase going from sodium to silicon, then decrease going to argon.
melting involves breaking up the lattice structure of a solid. hence, the melting point of an element indicates the strength of forces holding the particles together in the crystal lattice.
melting point increases from Na to Al as the strength of the metallic bond increases,
Si has a very high melting point due to its giant covalent structure. A large amount of energy is required to break the strong covalent bonds in Si.
P, S, Cl and Ar have lower melting points since they are simple molecules with weak van der Waals' forces between molecules.
state of sulfur:solid
TREND OF MELTING POINT FOR ELEMENTS IN PERIOD 3:
Melting points generally increase going from sodium to silicon, then decrease going to argon.
melting involves breaking up the lattice structure of a solid. hence, the melting point of an element indicates the strength of forces holding the particles together in the crystal lattice.
melting point increases from Na to Al as the strength of the metallic bond increases,
Si has a very high melting point due to its giant covalent structure. A large amount of energy is required to break the strong covalent bonds in Si.
P, S, Cl and Ar have lower melting points since they are simple molecules with weak van der Waals' forces between molecules.
Sunday, August 9, 2009
Energetics
DEFINITIONS:
Standard enthalpy change of reaction: Enthalpy change when molar quantities of reactants, as stated in the balanced stoichiometric equation, react together.
Standard enthalpy change of neutralisation: Enthalpy change when one mole of water is formed during the neutralisation between an acid and a base under standard conditions of 1atm and 298k.
Standard enthalpy change of formation: Enthalpy change when one mole of a pure compound is formed from its constituent elements in their standard states under standard conditions of 1 atm and 298k.
Standard enthalpy change of combustion: Enthalpy change when one mole of a substance is completely burnt in excess oxygen under standard conditions of 1atm and 298k.
Standard enthalpy change of atomisation: Enthalpy change when one mole of separate gaseous atoms are produced from the element in its standard state under standard conditions of 1 atm and 298k.
Standard enthalpy change of hydration: Enthalpy change when 1 mole of free gaseous ions is surrounded by water molecules and form a solution at infinite dilution, under standard conditions of 1atm and 298k.
Standard enthalpy change of solution: Enthalpy change when 1 mole of solute is completely dissolved in a solvent to form an infinitely dilute solution under standard conditions of 1 atm and 298k.
Bond dissociation energy: Energy required to break one mole of covalent bonds between 2 atoms in the gaseous state.
First ionisation energy: minimum energy required to remove one mole of electrons from one mole of gaseous atoms producing one mole of gaseous singly charged positive ions.
Electron affinity: Enthalpy change when one mole of gaseous atoms or negatively charged ion gain one mole of electrons.
Lattice energy: Enthalpy change when 1 mole of an ionic crystalline solid is formed from its separate gaseous ions under standard conditions of 1atm and 298k.
Standard enthalpy change of reaction: Enthalpy change when molar quantities of reactants, as stated in the balanced stoichiometric equation, react together.
Standard enthalpy change of neutralisation: Enthalpy change when one mole of water is formed during the neutralisation between an acid and a base under standard conditions of 1atm and 298k.
Standard enthalpy change of formation: Enthalpy change when one mole of a pure compound is formed from its constituent elements in their standard states under standard conditions of 1 atm and 298k.
Standard enthalpy change of combustion: Enthalpy change when one mole of a substance is completely burnt in excess oxygen under standard conditions of 1atm and 298k.
Standard enthalpy change of atomisation: Enthalpy change when one mole of separate gaseous atoms are produced from the element in its standard state under standard conditions of 1 atm and 298k.
Standard enthalpy change of hydration: Enthalpy change when 1 mole of free gaseous ions is surrounded by water molecules and form a solution at infinite dilution, under standard conditions of 1atm and 298k.
Standard enthalpy change of solution: Enthalpy change when 1 mole of solute is completely dissolved in a solvent to form an infinitely dilute solution under standard conditions of 1 atm and 298k.
Bond dissociation energy: Energy required to break one mole of covalent bonds between 2 atoms in the gaseous state.
First ionisation energy: minimum energy required to remove one mole of electrons from one mole of gaseous atoms producing one mole of gaseous singly charged positive ions.
Electron affinity: Enthalpy change when one mole of gaseous atoms or negatively charged ion gain one mole of electrons.
Lattice energy: Enthalpy change when 1 mole of an ionic crystalline solid is formed from its separate gaseous ions under standard conditions of 1atm and 298k.
Saturday, July 18, 2009
Gaseous State
Boyle's law: the volume of a fixed mass of gas is inversely proportional to its pressure, provided the temperature remains constant.
Charles's law: the volume of a fixed mass of gas is directly proportional to its absolute temperature, provided the pressure remains constant. When temperature increase, pressure increases.
Avogadro's law: under the same conditions of temperature and pressure, the volume of gas is proportional to the amount of gas present.
IDEAL GAS EQUATION: pV=nRT
R is always taken to be 8.31 JK-1mol-1
pressure: Pa
Volume: m3
temperature: K
Basic assumptions of the kinetic theory as applied to an ideal gas:
1)the volume of the gas particles is negligible compared to the volume occupied by the whole gas.
2)the intermolecular forces of attraction between the molecules of the gas are negligible.
3)gas particles are in constant random motion. they continually collide with each other and pressure arises from the bombardment of these particles.
4) collisions between particles of the gas are perfectly elastic.
Evidence of deviation from ideality:
-many gases are termed real gases and they obey the equation only approximately at low pressure and high temperature.
GASES DEVIATE FROM IDEALITY AT HIGH PRESSURES AND LOW TEMPERATURES.
GASES BEHAVE MORE IDEALLY WHEN THEIR MOLECULES ARE SMALL AND NON-POLAR AND AT LOW PRESSURES AND HIGH TEMPERATURES.
Charles's law: the volume of a fixed mass of gas is directly proportional to its absolute temperature, provided the pressure remains constant. When temperature increase, pressure increases.
Avogadro's law: under the same conditions of temperature and pressure, the volume of gas is proportional to the amount of gas present.
IDEAL GAS EQUATION: pV=nRT
R is always taken to be 8.31 JK-1mol-1
pressure: Pa
Volume: m3
temperature: K
Basic assumptions of the kinetic theory as applied to an ideal gas:
1)the volume of the gas particles is negligible compared to the volume occupied by the whole gas.
2)the intermolecular forces of attraction between the molecules of the gas are negligible.
3)gas particles are in constant random motion. they continually collide with each other and pressure arises from the bombardment of these particles.
4) collisions between particles of the gas are perfectly elastic.
Evidence of deviation from ideality:
-many gases are termed real gases and they obey the equation only approximately at low pressure and high temperature.
GASES DEVIATE FROM IDEALITY AT HIGH PRESSURES AND LOW TEMPERATURES.
GASES BEHAVE MORE IDEALLY WHEN THEIR MOLECULES ARE SMALL AND NON-POLAR AND AT LOW PRESSURES AND HIGH TEMPERATURES.
Friday, April 10, 2009
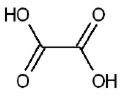
Formula of ethanedioic acid: HOOCCOOH·2H2O
Ethanedioic Acid (also called Oxaylic Acid) is a colourless, crystalline, toxic organic compound belonging to the family of dicarboxylic acids; melting at 187 C; soluble in water, alcohol, and ether. It is white crystals. It occurs in the form of its metal salts (usually calcium or potassium) in many plants. It is commercially manufactured by heating sodium formate in the presence of an alkali catalyst to form sodium oxalate, which should be converted to free oxalic acid when treated with sulfuric acid. It is also prepared by oxidizing carbohydrates with nitric acid, by heating saw dust with caustic alkalies or by fermentation of sugar solutions in the presence of certain molds. Oxalic acid is the only possible compound in which two carboxyl groups are joined directly; for this reason oxalic acid is one of the strongest acids in organic compounds. Unlike other carboxylic acids, oxalic acid (and formic acid) is readily oxidized and combine with calcium, iron, sodium, magnesium, or potassium to form less soluble salts called oxalates. Oxalic acid and oxalates are useful as reducing agents for photography, bleaching, and rust removal. They are widely used as an purifying agent in pharmaceutical industry, precipitating agent in rare-earth metal processing, bleaching agent in textile and wood industry, rust-remover for metal treatment, grinding agent, waste water treatment. acid rinse in laundries and removing scale from automobile radiators.
Saturday, March 28, 2009
Atomic Structure
What have i learnt(Atomic Structure)
-protons are positively charged(+1) and have a relative mass of 1
-neutrons are neutral(0) and have a relative mass of 1
-electrons are negatively charged(-1) and have a relative mass of 1/1840
The effect of magnetic field on beams of protons, neutrons and electrons.
Moving protons and electrons are deflected by a magnetic field. The protons will be deflected to the negative electrode and the electrons will be deflected to the positive electrode.
Isotopic: Atoms, ions or molecules containing same number of protons.
Isotonic: Atoms, ions or molecules containing same number of neutrons.
Isoelectronic: Atoms, ions or molecules containing same number of electrons.
Principal Types of sub-shells No. of electrons in each principal quantum Quantum Shell
1 s 2
2 s,p 8
3 s,p,d 18
4 s,p,d,f 32
Aufbau principle
Electrons occupy orbitals of the lowest energy available.
Pauli Exclusion Principle
Each orbital can accommodate only two electrons whose spins are always opposite.
Hund's Rule
When electrons are added successively to a subshell, they occupy the orbitals singly first before pairing occurs.
-protons are positively charged(+1) and have a relative mass of 1
-neutrons are neutral(0) and have a relative mass of 1
-electrons are negatively charged(-1) and have a relative mass of 1/1840
The effect of magnetic field on beams of protons, neutrons and electrons.
Moving protons and electrons are deflected by a magnetic field. The protons will be deflected to the negative electrode and the electrons will be deflected to the positive electrode.
Isotopic: Atoms, ions or molecules containing same number of protons.
Isotonic: Atoms, ions or molecules containing same number of neutrons.
Isoelectronic: Atoms, ions or molecules containing same number of electrons.
Principal Types of sub-shells No. of electrons in each principal quantum Quantum Shell
1 s 2
2 s,p 8
3 s,p,d 18
4 s,p,d,f 32
Aufbau principle
Electrons occupy orbitals of the lowest energy available.
Pauli Exclusion Principle
Each orbital can accommodate only two electrons whose spins are always opposite.
Hund's Rule
When electrons are added successively to a subshell, they occupy the orbitals singly first before pairing occurs.
Wednesday, March 18, 2009
My chosen career
My field of interest is medicine and i aspire to become a surgeon when i grow up.
My ambition to become a surgeon is strong as i have burning desire in me that makes me want to help the people around me in any way I can.
From what i have gathered, I have found out that Chemistry is necessary to join the medical course is university along wih either Physics or Biology.I realise that to enter medicine, good grades are vital especially in Chemistry, Physics and even Maths. The medical degree is one that requires a basic 4 year degree followed by 2 additional years to attain a doctorate in medicine.
My ambition to become a surgeon is strong as i have burning desire in me that makes me want to help the people around me in any way I can.
From what i have gathered, I have found out that Chemistry is necessary to join the medical course is university along wih either Physics or Biology.I realise that to enter medicine, good grades are vital especially in Chemistry, Physics and even Maths. The medical degree is one that requires a basic 4 year degree followed by 2 additional years to attain a doctorate in medicine.
My areas of weakness and strength in Chemistry.
My areas of weakness in Chemistry
So far, i have realised that I am not good at showing the relative proportions of the reactants and products involved in terms of the number of moles.
For eg.
MgC02 + 2HCl -> MgCl2 + CO2 +H2O
1 mole reacts with 2 mole to give 1 mole, 1 mole, 1 mole
or 0.2 moles, 0.4 moles, 0.2 moles, 0.2 moles
Another thing that i am not really good at is using the combustion data. The one where the formula given is CxHy(g) + (x+y/4)O2(g) -> xCO2(g) + y/2H2O(l)
My areas of strength in ChemistryThe areas that I am good at is the calculation part and im also good at balancing equations. Another part that i am good at is calculating the percentage yield.
Things that i learn from lab notes:
-the parts of a burette and pipette.
-how to handle the apparatus.
-before using any apparatus, i have to rinse them with distilled water.
-after rinsing th apparatus with distilled water, i have to rinse them with the original solution.
-i cannot rinse the conical flask with the original solution because this will result in an increase in amount of titrant used.
-i have to make sure that there is no air bubble at the tip of the burette.
BROMOTHYMOL BLUE; strong acid-strong base
PHENOLPHTHALEIN; weak acid-strong base
METHYL ORANGE; strong acid-weak base
-the parts of a burette and pipette.
-how to handle the apparatus.
-before using any apparatus, i have to rinse them with distilled water.
-after rinsing th apparatus with distilled water, i have to rinse them with the original solution.
-i cannot rinse the conical flask with the original solution because this will result in an increase in amount of titrant used.
-i have to make sure that there is no air bubble at the tip of the burette.
BROMOTHYMOL BLUE; strong acid-strong base
PHENOLPHTHALEIN; weak acid-strong base
METHYL ORANGE; strong acid-weak base
Monday, March 2, 2009
Atoms, Moles and Stoichiometry
Things that i learn from atoms, moles and stoichiometry:
-SI base units(e.g. metre, kilogram, second)
-Prefixes(giga, kilo, mega, deci, centi)
-how to determine the number of significant figures
-
-SI base units(e.g. metre, kilogram, second)
-Prefixes(giga, kilo, mega, deci, centi)
-how to determine the number of significant figures
-
Subscribe to:
Comments (Atom)